ANODES FOR SHAFTS, KEELS, AND PROPELLERS
Anodes for FLEXOFOLD propellers.
Cone spiral
Anodes for Max-Prop
Volvo Sail Drive Foot Ring with Max-Prop propeller.
ROUND WARPING CONE MODEL RIVA H.86 DIAM.116
WARHEAD FOR BRAVO 3 MERCURY- MERCRUISER
Volvo Sail Drive foot ring with Max-Prop propeller.
Stern anode for cap. 235x80x40 F.14
Day and night visual signal table COLREG72Cathodic protection anode designed for marine applications, ideal for combating galvanic corrosion on hulls and metal components. Manufactured to high quality standards, it is part of the CAP series of the Tecnoseal brand, a leader in cathodic protection.
Characterised by a robust design with a central hole for simple and secure fastening.
Dimensions: 235 x 80 mm, height 40 mm, 1 mounting hole.
Oval zinc plate 150x60x60 cf.38.1
The Tecnoseal oval zinc plate is a sacrificial anode designed to protect hulls, rudders, and other submerged metal components from the corrosive action of water. With dimensions of 150x60x60 mm and a 38.1 mm center hole, it provides effective protection against galvanic corrosion, prolonging the life of metal parts.
Made from high-quality zinc, this anode is ideal for boats and marine structures operating in salt water. Reliability, durability and optimal performance are the hallmarks of Tecnoseal products, leaders in the cathodic protection industry.
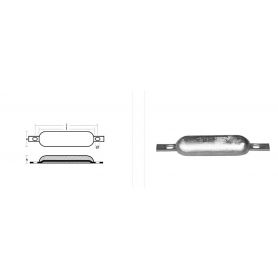
Oval anode with slotted insert c.f. 250 KG.3
This anode, part of the 00300A Series, is designed for marine applications requiring effective cathodic protection. Its oval shape and convenient slotted insert allow for easy and secure bolt-on installation.
The alloy used meets the "c.f. 250 kg. 3" classification, ensuring excellent protection performance and durability even under demanding operating conditions.
Exact dimensions of the anode are provided in the attached photo, allowing for a targeted selection based on specific installation needs.
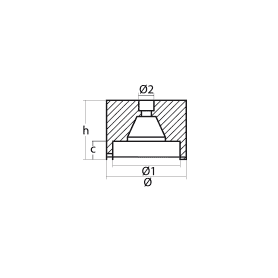
OGIVE ROOT REQ. AXIS DIA 25
HEXAGONAL ROOT WARHEAD, REQ. AXIS D.25
Ogiva Sole' D.25mm
The Solé Ø25 ogive from Tecnoseal is a high-quality sacrificial anode designed to effectively protect the propeller and drive shaft from galvanic currents and electrolytic corrosion.
Made of zinc, aluminum, or magnesium alloy (depending on the type chosen and the environment of use—sea, fresh water, or brackish water), it guarantees high cathodic protection, extending the life of the immersed metal components. The 25 mm diameter is suitable for propeller shafts of that size, ensuring precise and secure installation.